Categories
- Balance (11)
- Book (5)
- Chromatography (21)
- Clinical Trial (14)
- Engineering (49)
- FDA (4)
- Formulation Development (47)
- HPLC (20)
- Human Resource (14)
- Microbiology (51)
- Packing (26)
- Parenteral (5)
- Pharmacist (55)
- Pharmacoeconomics (44)
- Production (104)
- Qualification (4)
- Quality Assurance (80)
- Quality Control (206)
- Regulatory Affair (47)
- Validation (19)
- Warehouse (43)

A Guide to the Pharmaceutical Industry Insurance Policy
November 02, 2024

SOP for Operation of Shimadzu HPLC LC-2010 CHT
October 07, 2023

Guide to Pharmaceutical Insurance in the United States
November 02, 2024

Pharmaceutical Trading: What is it and How to Do it?
November 02, 2024
.webp)
SOP for Calibration of Shimadzu HPLC (Prominence – i LC – 2030)
December 10, 2023

Online F1 and F2 Calculator
May 31, 2025

SOP for Excel Sheet Validation in Pharmaceuticals
September 17, 2023
.webp)
SOP for Operation of Dissolution Test Apparatus (Make: LAB INDIA)
October 08, 2023
CTD vs. eCTD Submission Formats
PharmaInfo
June 25, 2025
In pharmaceuticals and biotechnology, understanding submission formats is crucial for successful product approvals. Two key formats dominate this space: the Common Technical Document (CTD) and its digital evolution, the Electronic Common Technical Document (eCTD). While both serve the same foundational purpose—streamlining regulatory submissions across global markets—their differences can significantly impact your submission strategy. Let’s explore what sets CTD and eCTD apart, and why moving toward eCTD is increasingly non-negotiable.
What is CTD?
The Common Technical Document (CTD) can be electronically submitted by the applicant to a regulator, like the USFDA or EMA, thanks to the Electronic Common Technical Document (eCTD). The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) established the Common Technical Document, which outlines the structure of the modules, sections, and documents that an applicant for a marketing authorization for a medicinal product for human use must use.
The Common Technical Document (CTD) is a harmonized submission format agreed upon by the International Council for Harmonization (ICH). It organizes data into five standardized modules:
- Administrative and product information
- Summaries
- Quality
- Nonclinical study reports
- Clinical study reports
This format was a game-changer when introduced, helping regulatory authorities and applicants reduce duplication of effort and streamline global submissions.
What is eCTD?
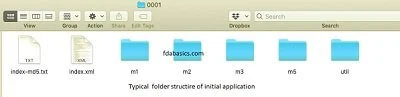
The Electronic Common Technical Document (eCTD) builds on the CTD structure but offers a fully digital, more efficient method for managing submissions. At its core, eCTD is a system of PDF and XML files organized in a standardized folder structure, accessible via an XML backbone and verified with MD5 checksums for data integrity.
ALSO READ: Regulatory Requirements of EU & MHRA
This electronic format is now mandatory in many regions, including the United States and Europe, for nearly all types of product submissions—ranging from investigational applications to marketing authorizations.
eCTD is a harmonized regulatory filing format widely accepted by several countries. ICH adopted countries have common modules 2, 3, 4, and 5 and a customized administrative module 1 according to country-specific requirements.
Contents of e-CTD
- ICH recommended the CTD folder structure
- XML backbone containing metadata such as document id, document version, hyperlinks, file title, and leaf operation
- Regional module XML backbone, files, and folders (such as application form and cover letter)
- Documents in the Word, pdf, xpt, or other agency-recommended format
- Checksum values for documents and XML backbone
Key Components of eCTD
An eCTD submission comprises:
- Directory structure: A predefined hierarchy of folders
- XML eCTD instance: The navigational map for reviewers
- Content files: Typically PDFs, occasionally XMLs, and graphics (preferably in PDF format)
In the U.S., the FDA provides specific guidelines for formatting Modules 1–5, ensuring consistency and compliance. The EMA follows a similar structure within the EU, with additional harmonized technical guidelines for electronic submissions.
Regulatory Use Cases
In the U.S., eCTD is required for:
- Certain Investigational New Drug Applications (INDs)
- New Drug Applications (NDAs)
- Abbreviated NDAs (ANDAs)
- Certain Biologics License Applications (BLAs)
Exemptions include:
- Expanded access INDs for individual patients
- Emergency use protocols
- Intermediate-sized patient population treatments
In the EU, eCTD is mandatory for:
- All types of submissions for Marketing Authorization
- Centralized, Decentralized, and Mutual Recognition Procedures
- Products treating critical conditions like HIV, cancer, diabetes, and rare/orphan diseases
Why Switch to eCTD?
- Regulatory Compliance: Major authorities now require eCTD.
- Efficiency: Faster processing, easier navigation, and streamlined updates.
- Data Management: Improved tracking and lifecycle management of documents.
- Global Acceptance: eCTD is a harmonized format recognized across ICH regions.
Advantages of eCTD
- Regulatory life cycle tracking at both application and document levels.
- Document integrity is verified through a checksum, which acts as a fingerprint—any change in the document results in a different checksum. For ease of review, agencies can quickly search for required information, and hyperlinking facilitates navigation to cross-linked data.
- Applicants can easily access links to information from previous submissions. This system helps applicants efficiently replace, add, or delete documents during review. agencies can easily track.
How to prepare eCTD?
The International Conference on Harmonization (ICH) has released harmonized eCTD specifications for Modules 2 through 5. Module 1 guidelines are determined by individual country agencies. Several software options are available for eCTD publishing, and it is possible to publish without specialized software; however, this process is complex and requires XML editing skills. Before publishing, you may need to insert bookmarks, create a table of contents, add hyperlinks, and prepare PDF documents in accordance with regulatory agency standards.
As regulatory authorities continue to modernize and digitize submission processes, the eCTD format is no longer optional—it’s essential. Understanding the differences between CTD and eCTD is the first step toward streamlined, compliant, and successful global submissions.
ALSO READ: CTD, eCTD, and Their Various Modules
Popular Posts
New Drug Application (NDA) Process
June 06, 2023
Drug Approval Process in Japan
June 29, 2023
SOP for Handling of Deviation
October 14, 2023
Drug Approval Process in Europe
May 30, 2023
Effective Dossier Management in Regulatory Affairs
June 26, 2023
SOP for Operation of Shimadzu HPLC (Prominence – i LC – 2030)
October 07, 2023
Role of FDA in Drug Development Process
June 06, 2023
Most Popular
SOP for Numbering System of Analytical Report Number
November 16, 2023
A Guide to the Pharmaceutical Industry Insurance Policy
November 02, 2024
SOP for Operation of Shimadzu HPLC LC-2010 CHT
October 07, 2023
Footer Menu Widget
Copyright © 2026 PharmaInfo All Right Reserved.
Created By Blogspot Theme | Distributed By Gooyaabi Templates









0 Comments