Categories
- Balance (11)
- Book (5)
- Chromatography (21)
- Clinical Trial (14)
- Engineering (49)
- FDA (4)
- Formulation Development (47)
- HPLC (20)
- Human Resource (14)
- Microbiology (51)
- Packing (26)
- Parenteral (5)
- Pharmacist (55)
- Pharmacoeconomics (44)
- Production (104)
- Qualification (4)
- Quality Assurance (80)
- Quality Control (206)
- Regulatory Affair (47)
- Validation (19)
- Warehouse (43)

SOP for Operation of Shimadzu HPLC LC-2010 CHT
October 07, 2023

A Guide to the Pharmaceutical Industry Insurance Policy
November 02, 2024

Pharmaceutical Trading: What is it and How to Do it?
November 02, 2024

Guide to Pharmaceutical Insurance in the United States
November 02, 2024
.webp)
SOP for Calibration of Shimadzu HPLC (Prominence – i LC – 2030)
December 10, 2023

SOP for Excel Sheet Validation in Pharmaceuticals
September 17, 2023
.webp)
SOP for Operation of Dissolution Test Apparatus (Make: LAB INDIA)
October 08, 2023

Online F1 and F2 Calculator
May 31, 2025
Drug-Excipient Compatibility Study in Pharmaceutical Formulation
PharmaInfo
May 17, 2025
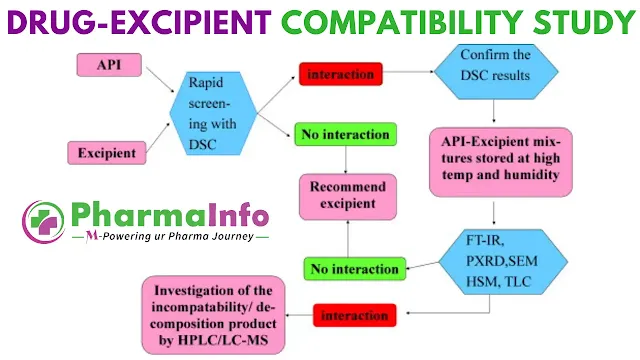
In the fast-paced world of pharmaceutical development, ensuring that an active pharmaceutical ingredient (API) remains stable and effective throughout its shelf life is non-negotiable. Drug-excipient compatibility studies (DECS) lie at the heart of this endeavor, providing critical insights into how a drug interacts with the various inactive ingredients (excipients) that compose the final dosage form. Far from being a mere regulatory checkbox, DECS forms a strategic tool—one that can prevent costly reformulations, avert clinical surprises, and ultimately accelerate time-to-market for innovative therapies.
Objectives of DECS
At its core, DECS aims to:
- Identify compatible excipients: Selecting the right excipients is not a matter of convenience; it defines the therapeutic efficacy, bioavailability, and patient acceptability of the final product.
- Optimize stability: By pre-screening drug-excipient blends under stress conditions, formulators can maximize the shelf life of solid and liquid dosage forms.
- Inform IND submissions: Regulatory bodies such as the US FDA now mandate submission of DECS data for Investigational New Drug (IND) applications, underscoring its critical role in early-stage development
- Guide formulation strategy: DECS data steer decisions on excipient selection, dose form type, and storage conditions—factors that shape the commercial and clinical success of a molecule.
Understanding Incompatibility
Incompatibilities fall into three principal categories:
- Physical incompatibility: Manifested as color changes, liquefaction, caking, or phase separation when the drug and excipient are combined.
- Chemical incompatibility: Involves the formation of new, undesirable compounds via hydrolysis, oxidation, reduction, decarboxylation, racemization, or other chemical transformations.
- Therapeutic incompatibility: An in vivo phenomenon where the presence of an excipient alters the drug’s pharmacokinetic or pharmacodynamic profile .
Failing to detect such interactions early can result in decreased potency, off-odors, patient safety risks, or outright formulation failure. For example, primary amines readily react with lactose to produce colored Schiff bases—an easily spotted warning sign, yet one that can devastate commercial product stability if overlooked.
Compatibility Testing: From Solid to Liquid
DECS encompass both solid-state and liquid-state evaluations:
Solid-State Reactions
- Sample Preparation: Binary mixtures of drug and excipient (typically 1:1 by weight) are sealed in vials, often under controlled moisture levels (e.g., 5% w/w) to simulate real-world humidity stresses.
- Stress Conditions: Samples are stored at various temperatures, humidity levels, and light intensities for 1–12 weeks. Observations include caking, liquefaction, discoloration, odor development, or gel formation.
- Assays: Analytical techniques such as high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), and differential scanning calorimetry (DSC) quantify degradation products. Advanced methods like mass spectrometry and nuclear magnetic resonance (NMR) may identify unknown impurities.
Liquid-State Reactions
- Stressors: Formulations are tested under acidic/alkaline pH, oxidative and reductive atmospheres (pure oxygen or nitrogen), and in the presence of stabilizers or chelating agents.
- Formulation Additives: Common excipients in solutions or suspensions, such as ethanol, glycerin, sucrose, preservatives, and buffers, are evaluated for their impact on API stability.
- Detection: Changes in potency or appearance are monitored via HPLC, UV-Vis spectroscopy, and visual inspection, often accelerated by autoclaving or light exposure tests.
ALSO READ: SOP for Drug-Excipient Compatibility Studies
Analytical Techniques: Strengths and Limitations
A robust DECS protocol leverages a complementary suite of analytical methods:
- Differential Scanning Calorimetry (DSC): Rapid and sensitive to thermal events, DSC pinpoints melting point shifts and endothermic/exothermic transitions indicative of interactions. Yet, it may miss slow-forming incompatibilities that only emerge during long-term storage, necessitating orthogonal methods like FT-IR or TLC for confirmation.
- Differential Thermal Analysis (DTA): Similar to DSC but focused on temperature differentials between a sample and inert reference, DTA helps rank excipient suitability (e.g., Avicel vs. spray-dried lactose for enalapril maleate formulations) by correlating interaction onset with projected shelf life.
- Accelerated Stability Studies: Exposing multiple formulations to elevated temperature (e.g., 40°C) and relative humidity (75% RH) offers rapid insight into degradation kinetics. Plotting percent drug remaining versus time identifies the excipient combination that maximizes API stability.
- Spectroscopic Methods (FT-IR, DRS)
- FT-IR detects specific bond changes (e.g., carbonyl shifts) in dried mixtures.
- Diffuse Reflectance Spectroscopy (DRS) excels at identifying surface discoloration from oxidation or Maillard reactions in powders.
- Chromatography (TLC, HPTLC, HPLC)
- TLC/HPTLC serve as quick qualitative checks: intact peaks for drug and excipient indicate compatibility, whereas new RF values signal complex formation.
- Self-Interactive Chromatography (SIC) immobilizes the drug on the stationary phase to probe excipient affinity via retention time shifts, particularly useful for protein-based drugs.
- Miscellaneous Techniques: Radiolabelled assays, vapor pressure osmometry, and fluorescence spectroscopy round out the toolkit, each providing unique sensitivity to specific interaction types.
Case Studies: Learning from Practice
- Ofloxacin–Lactose Incompatibility (DSC): A physical mixture exhibited complete disappearance of the ofloxacin melting peak at 278 °C, immediately flagging an interaction. In contrast, starch and talc blends showed slight onset shifts, deeming them acceptable carriers.
- Ascorbic Acid Formulations: Under nitrogen vs. air atmospheres, binary mixtures with microcrystalline cellulose (MCC) and ion-exchange excipients demonstrated shifts of 50–100 °C in thermal degradation onset—critical data for optimizing tablet storage conditions.
- Theophylline Hydrate Formation: During wet granulation, lactose monohydrate exacerbated pseudopolymorphic changes, slowing dissolution. Silicified MCC, however, inhibited hydrate formation, preserving rapid drug release.
- Aerosol Compatibility: Propellant-11 (trichloromonofluoromethane) generates HCl upon decomposition, corroding aluminum containers—a stark reminder that excipient-container interactions can be as consequential as drug-excipient ones.
Known Incompatibilities and Impurities
A forward-thinking formulator recognizes that excipient quality—even at trace levels—can make or break a product:
- Impurities in Dicalcium Phosphate (DCP): Iron levels above 0.04% catalyze the degradation of meclizine hydrochloride.
- Hydroperoxides in Polymers: Povidone, PEG-400, and polysorbate-80 frequently harbor hydroperoxides that oxidize sensitive APIs unless rigorously monitored.
- Gelatin Shell Iron Migration: In soft-gelatin capsules, iron migration from fill solutions can darken the shell and compromise aesthetic quality.
Moreover, certain excipients—such as polysorbate-80—can inhibit P-glycoprotein efflux pumps, unintentionally boosting cellular uptake and altering drug pharmacokinetics. Awareness of these bio-interactions is paramount in today’s precision-medicine era.
Practical Recommendations
- Early Screening: Implement DECS at the preformulation stage using small sample sizes (5–10 mg) under inert atmospheres to filter out incompatible excipients.
- Orthogonal Confirmation: Never rely on a single analytical method. Pair thermal analysis with spectroscopy and chromatography for holistic insight.
- Excipient Quality Control: Source excipients from reputable suppliers who provide impurity profiles, especially hydroperoxide and heavy-metal content.
- Container Compatibility: Extend compatibility testing to packaging materials and propellants, recognizing their potential to introduce degradation pathways.
- Future Directions: Embrace high-throughput DECS platforms and computational predictive models to accelerate formulation design with minimal material consumption.
Conclusion
Drug-excipient compatibility studies are much more than a regulatory formality; they are a strategic asset in modern pharmaceutical development. By integrating rigorous DECS protocols, leveraging complementary analytical techniques, and maintaining vigilant quality control of excipients, formulators can avert downstream surprises, optimize drug performance, and deliver safer, more effective therapies to patients. As the complexity of APIs and delivery platforms continues to grow, so too must our commitment to forward-looking compatibility strategies—because in the quest for pharmaceutical innovation, there is no substitute for knowing exactly how every component plays its part.
Popular Posts
New Drug Application (NDA) Process
June 06, 2023
Drug Approval Process in Japan
June 29, 2023
SOP for Handling of Deviation
October 14, 2023
Drug Approval Process in Europe
May 30, 2023
Effective Dossier Management in Regulatory Affairs
June 26, 2023
SOP for Operation of Shimadzu HPLC (Prominence – i LC – 2030)
October 07, 2023
Role of FDA in Drug Development Process
June 06, 2023
Most Popular
SOP for Numbering System of Analytical Report Number
November 16, 2023
SOP for Operation of Shimadzu HPLC LC-2010 CHT
October 07, 2023
A Guide to the Pharmaceutical Industry Insurance Policy
November 02, 2024
Footer Menu Widget
Copyright © 2026 PharmaInfo All Right Reserved.
Created By Blogspot Theme | Distributed By Gooyaabi Templates







0 Comments